Order online by selecting the assay of your choice and add to cart. Upon receipt a Seegene Sales representative will follow up to complete your order request.
-
Analytes
Allplex™ 2019-nCoV Assay* (EUA)
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- SARS-CoV-2 (E gene)
- SARS-CoV-2 (RdRP gene)
- SARS-CoV-2 (N gene)
-
Analytes
Novaplex™ PneumoBacter Assay
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Bordetella parapertussis (BPP)
- Bordetella pertussis (BP)
- Chlamydophila pneumoniae (CP)
- Haemophilus influenzae (HI)
- Legionella pneumophila (LP)
- Mycoplasma pneumoniae (MP)
- Streptococcus pneumoniae (SP)
-
Analytes
Novaplex™ Respiratory Panel 1A
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Flu A-H1
- Flu A-H1pdm09
- Flu A-H3
- Influenza A virus (Flu A)
- Influenza B virus (Flu B)
- Respiratory syncytial virus A (RSV A)
- Respiratory syncytial virus B (RSV B)
-
Analytes
Novaplex™ Respiratory Panel 2
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Adenovirus (AdV)
- Enterovirus (HEV)
- Metapneumovirus (MPV)
- Parainfluenza virus 1 (PIV 1)
- Parainfluenza virus 2 (PIV 2)
- Parainfluenza virus 3 (PIV 3)
- Parainfluenza virus 4 (PIV 4)
-
Analytes
Novaplex™ Respiratory Panel 3
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Bocavirus 1/2/3/4 (HBoV)
- Rhinovirus A/B/C (HRV)
- Coronavirus NL63 (CoV NL63)
- Coronavirus 229E (CoV 229E)
- Coronavirus OC43 (CoV OC43)
-
Analytes
Novaplex™ RV Master
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Human adenovirus (AdV)
- Human metapneumovirus (MPV)
- Human parainfluenza virus (PIV)
- Human respiratory syncytial virus (RSV)
- Human rhinovirus (HRV)
- Influenza A virus (Flu A)
- Influenza B virus (Flu B)
- SARS-CoV-2 (N gene)
- SARS-CoV-2 (RdRP gene)
- SARS-CoV-2 (S gene)
-
Analytes
Novaplex™ SARS-CoV-2/FluA/FluB/RSV
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- SARS-CoV-2 (RdRP gene)
- SARS-CoV-2 (N gene)
- SARS-CoV-2 (S gene)
- Respiratory syncytial virus A/B (RSV A/B)
- Influenza A virus (Flu A)
- Influenza B virus (Flu B)
Nasopharyngeal swab
Nasopharyngeal aspirate
Bronchoalveolar lavage (BAL)
Oropharyngeal (throat) swab
Saliva
Sputum (Respiratory Panel 4 only)
Cultured cells
Fresh tissue
Automated Extraction & PCR Setup
Seegene NIMBUS
Seegene STARlet
Real-time PCR
CFX96™ Real-Time PCR Detection System (Bio-Rad)
CFX96™ Dx System (Bio-Rad)
CFX96™ Opus (Bio-Rad)
CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad)
Seegene Viewer Software
- Quick and easy data analysis and interpretation
- Interface specialized for multiplex testing
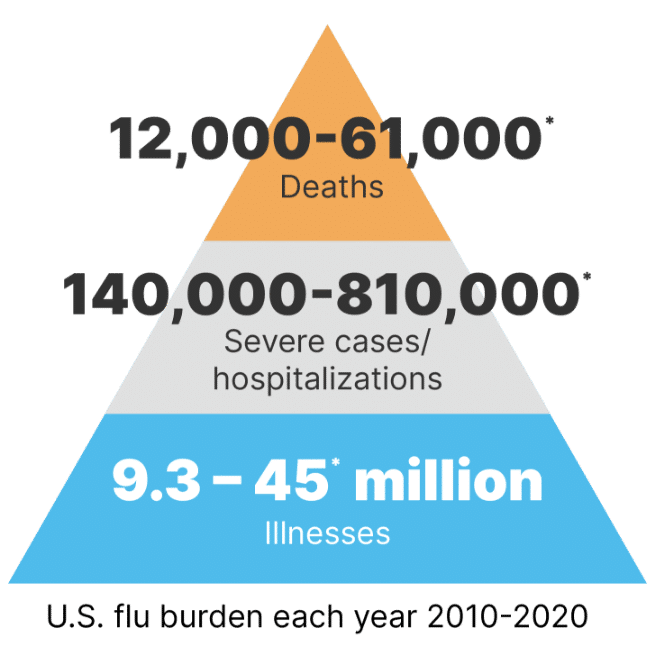
In the United States, influenza, pneumonia, and respiratory syncytial virus (RSV) are projected to infect more than 13 million people annually, with an associated mortality burden of 69,000 to nearly 100,000 deaths attributable to just influenza in the 2017–2018 season alone.2 With the new addition of SARS-CoV-2 the number of respiratory infections will continue to surge, and the US will maintain high infection rates annually.
Inflammation in various parts of the respiratory tract can be caused by respiratory pathogens, such as viruses and bacteria. Viral infections result from viruses that are inhaled, ingested, or that come into contact with the nasal area and occur through communal transmission. Some viruses such as parainfluenza, RSV, and metapneumovirus can cause lower respiratory infections. Our assays detect the most prevalent respiratory viruses that can be challenging to differentiate between.
Novaplex™ Respiratory Panel Assays* simultaneously detects a broad range of viruses and bacteria from a single test, providing more effective and efficient results. These assays are multiplex real-time PCR assays that detect and identify 16 viruses, 3 Flu subtypes and 7 bacteria in patient’s specimens. Up to 4 panels can be run in unison offering flexible testing options, maximizing throughput while reducing time to result.
Allplex™ 2019-nCoV Assay (Emergency Use Authorized)** is a multiplex real-time PCR product that can detect 3 target genes of SARS-CoV-2: N gene, RdRP gene, and S gene. While E gene is for the sarbecovirus group that includes both SARS-CoV and SARS-CoV-2.
Novaplex™ SARS-CoV-2/FluA/FluB/RSV Assay* is a multiplex real-time PCR assay designed to detect influenza A, influenza B and respiratory syncytial virus A/B, SARS-CoV-2 N , S , and RdRP gene in a single tube.
Novaplex™ Respiratory Panel 1A* Multiplex real-time RT-PCR system for detection of Influenza A virus, Influenza B virus, Human respiratory syncytial virus A, Human respiratory syncytial virus B, and subtyping of Influenza A virus (Human Influenza A virus subtype H1, H3, and H1pdm09).
Novaplex™ Respiratory Panel 2* is a multiplex real-time RT-PCR system for detection of Human adenovirus, Human metapneumovirus, Human enterovirus, Human parainfluenza virus 1, Human parainfluenza virus 2, Human parainfluenza virus 3, and Human parainfluenza virus 4.
Novaplex™ Respiratory Panel 3* is a multiplex real-time PCR system for detection of Human bocavirus 1/2/3/4, Human rhinovirus A/B/C, Human coronavirus 229E, Human coronavirus NL63, and Human coronavirus OC43.
Novaplex™ PneumoBacter Assay* is a multiplex real-time PCR system for detection of Chlamydophila pneumoniae, Mycoplasma pneumoniae, Legionella pneumophila, Bordetella pertussis, Bordetella parapertussis, Streptococcus pneumoniae, and Haemophilus influenzae.
Novaplex™ RV Master Assay* is a multiplex real-time RT-PCR assay for the detection of the SARS-CoV-2 E, RdRP, S, N genes, and the S gene variants associated with HV69/70del, Y144del, E48K, E48K, N501Y, P681H.
*Novaplex™ Assays are for Research Use Only. Not for use in diagnostic procedures.
**Allplex™ 2019-nCoV Assay is for Emergency Use Authorization.







