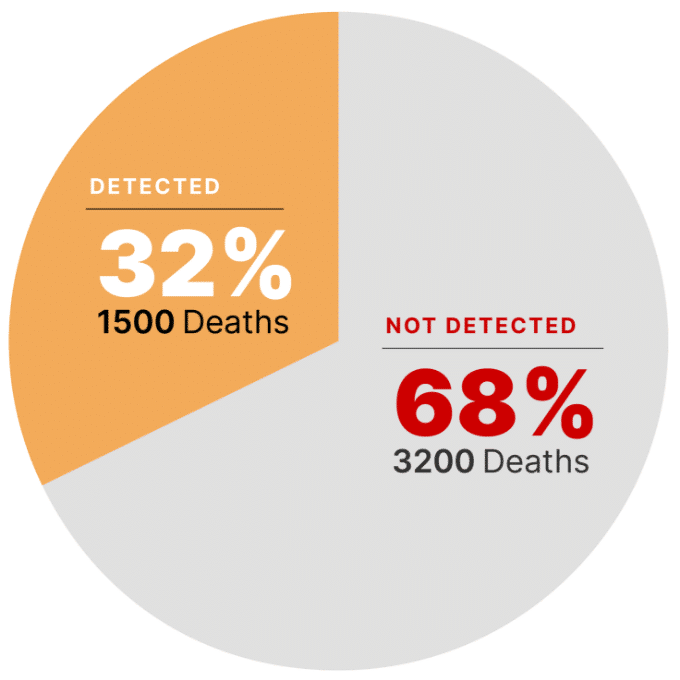
Gastrointestinal infections (food poisoning) caused by Salmonella and Listeria cause 1500 deaths annually in US. In fact, 3200 deaths are attributed by unidentified pathogens.1
Login here to access our database to find Instructions for Use and Safety Data Sheets. Don’t Have a password? Register here to receive one.
-
Analytes
Novaplex™ GI-Bacteria (I) PCR Assay
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Shigella spp./EIEC (Sh/EI)
- Vibrio spp. (Vib)
- Aeromonas spp. (Aer)
- Campylobacter spp. (Cam)
- Y. enterocolitica (Yer)
- C. difficile toxin A (TcdA)
- C. difficile toxin B (TcdB)
- Salmonella spp. (Sal)
- C. perfringens
-
Analytes
Novaplex™ GI-Bacteria (II) Assay
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Shiga toxin (stx1/2)
- E. coli O157 (O157)
- Hypervirulent C. difficile (CD hyper)
- EPEC (eaeA)
- ETEC (lt/st)
- EAEC (aggR)
-
Analytes
Novaplex™ GI-Helminth(I) Assay
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Enterocytozoon spp. / Encephalitozoon spp. (EN)
- Hymenolepis spp. (HY)
- Taenia spp. (TA)
- Enterobius vermicularis (EV)
- Strongyloides spp. (ST)
- Ascaris spp. (AS)
- Trichuris trichiura (TT)
- Ancylostoma spp. (AN)
- Necator americanus (NA)
-
Analytes
Novaplex™ GI-Parasite Assay
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Blastocystis hominis (BH)
- Dientamoeba fragilis (DF)
- Cyclospora cayetanensis (CC)
- Giardia lamblia (GL)
- Entamoeba histolytica (EH)
- Cryptosporidium spp. (CR)
-
Analytes
Novaplex™ GI-Virus Assay
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Adenovirus F (ADV-F)
- Astrovirus (ASV)
- Norovirus GI (NVG1)
- Norovirus GII (NVG2)
- Sapovirus (SV)
- Rotavirus A (ROV)
Stool
Automated Extraction & PCR Setup
Seegene NIMBUS
Seegene STARlet
Real-time PCR
CFX96™ Dx System
CFX96™ Opus (Bio-Rad)
CFX96 Touch™ Real-Time PCR Detection System
Seegene Viewer Software
- Quick and easy data analysis and interpretation
- Interface specialized for multiplex testing
![iStock-1288309877 [Converted]](https://seegeneus.com/wp-content/uploads/2023/03/iStock-1288309877-Converted.png)
Gastroenteritis is a very common infection, with more than 3 million cases per year in the US. It can be caused by a wide range of bacterial, viral, and/or parasitic pathogens. With gastroenteritis, the bowels, stomach, and intestines become irritated and inflamed leading to diarrhea, vomiting and nausea that can last for days or even weeks. Detection based on conventional methods has several drawbacks including low sensitivity, significant hands-on-time, and long turnaround time. This includes stool processing steps to prepare for microscopy that require specialized expertise for the detection of specific infection-causing ova and parasites.
Recently, multiplex real-time PCR-based syndromic testing has been at the forefront of molecular testing due to its many advantages such as simultaneous detection and broad coverage of gastrointestinal (GI) tract pathogens, short turnaround time, and a high reproducibility rate.
The Novaplex™ Gastrointestinal Panel Assays* are multiplex, real-time RT-PCR assays that detect and identify 34 GI pathogens including 6 viruses, 7 bacteria (I), 6 bacteria (II), 6 parasites, and 9 helminths simultaneously.
*Novaplex™ Assays are For Research Use Only. Not for Use in Diagnostic Procedures.
FEB.15.2023
This webinar discussed different methods of diagnosing parasites, contrasting microscopic approaches with novel diagnostic testing alternatives.
Parasites are recognized as important gastrointestinal pathogens with significant patient, economic, environmental, and public health impacts in the US. Currently, clinical laboratory diagnosis is reliant on microscopic methods even with novel diagnostic testing alternatives available. Common and undesirable outcomes from this approach include delayed results, high labor costs, inefficiency in the laboratory operation, and questions on quality. Issues of quality may arise from technologist shortages and lack of skilled expertise in microscopy and morphology. This can lead to errors in diagnosis and patient care. Novel methods may lack adequate coverage of the potential parasitic pathogens while sample types may be limited or difficult to process and test. As a result, labs often choose to send out for these tests.
- Pathologists
- Lab supervisors
- Lab directors (and/or assistant directors)
- Lab managers (supervisory and/or non-supervisory)
- Other laboratory professionals overseeing/conducting within this topic
At the end of this activity, participants will be able to:
- Explain the staffing challenges associated with traditional ova & parasite exams.
- Accurately describe new tools available to clinical labs for the diagnosis and screening of stool parasite testing.
- Evaluate the potential value of molecular testing for ova & parasite exams.
Richard Bradbury
PhD, FFSc RCPA, FASM, FASCTM
Senior Lecturer in Microbiology and Molecular Biology,
Federation University
Melbourne, Victoria
Australia
Susan Butler-Wu
PhD, D(ABMM), SM(ASCP)
Director, Clinical Microbiology, LAC+USC Medical Center
Associate Professor, Clinical Pathology, Keck School of Medicine of USC
Los Angeles, CA
Blaine A. Mathison
BS, M(ASCP)
Scientist III, ARUP Laboratories
Adjunct Instructor, University of Utah, Department of Pathology
Salt Lake City, UT
1 https://emedicine.medscape.com/article/175569-overview





