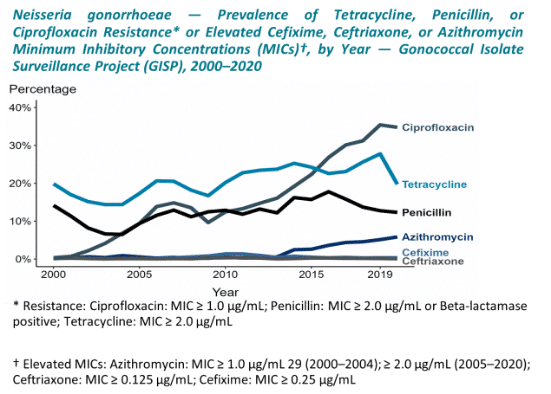
Antimicrobial resistance in gonorrhea has increased rapidly in recent years and has reduced the options for treatment in the US. Estimates of resistance to fluoroquinolones drug exceed 30%.1
-
Analytes
Novaplex™ MG & AziR
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Mycoplasma genitalium (MG)
- A2058G (mutation of 23S rRNA)
- A2058T (mutation of 23S rRNA)
- A2058C (mutation of 23S rRNA)
- A2059G (mutation of 23S rRNA)
- A2059T (mutation of 23S rRNA)
- A2059C (mutation of 23S rRNA)
-
Analytes
Novaplex™ MG & MoxiR
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- Mycoplasma genitalium (MG)
- G248A (mutation of parC)
- A247C (mutation of parC)
- G248T (mutation of parC)
- G259A (mutation of parC)
- G259C (mutation of parC)
- G259T (mutation of parC)
-
Analytes
Novaplex™ NG & DR
Select the assays you are interested in and a Seegene representative will contact you to complete your order.- A2059G (mutation of 23S rRNA)
- C2611T (mutation of 23S rRNA)
- S91F (mutation of gyrase A)
- Neisseria gonorrhoeae (NG1)
- Neisseria gonorrhoeae (NG2)
Urine
Genital swab
Liquid based cytology
A2059C (mutation of 23S rRNA)
Urine
Genital swab
Liquid based cytology
Urine
Genital swab
Oropharyngeal swab
Anorectal swab
Liquid based cytology
Automated Extraction & PCR Setup
Seegene NIMBUS
Seegene STARlet
Real-time PCR
CFX96™ Real-time PCR Detection System (Bio-Rad)
CFX96™ Dx System (Bio-Rad)
CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad)
Seegene Viewer Software
Quick and easy data analysis and interpretation
Interface specialized for multiplex testing
Novaplex™ MG & AziR Assay “Azithromycin resistance” and Novaplex™ MG & MoxiR Assay “Moxifloxacin”
- Simultaneous detection and identification of MG and mutations responsible for resistances of azithromycin (6 mutations) and moxifloxacin (6 mutations)
- Informative data with identification of each mutation related to drug resistances
- Short TAT (4 hours) from extraction to the final results
Novaplex™ NG &DR Assay
- Detects NG and drug resistance (Azithromycin, Ciprofloxacin) accurately with a single test to enable informative decision making
- Azithromycin-resistance (A2059G, C2611T
- Ciprofloxacin-resistance (S91F)
- Fast confirmation: Detects NG and drug resistance simultaneously with fast PCR TAT of 1.5 hours
- Combination test: Combination test available with Seegene’s STI assay (Novaplex CT/NG/MG/TV Assay) for informative STI testing
Neisseria gonorrhoeae (NG) is a major causative pathogen of STI and is one of the most commonly detected pathogens due to its high infection rate. NG can spread easily, but it is often asymptomatic and can be left untreated. When left untreated, NG can cause serious health problems in women including ectopic pregnancy and infertility. Treatment of gonorrhea has become more complex with increasing resistance to fluoroquinolone drugs and the need for monitoring and controlling other options like macrolide or cephalosporin resistance.
Novaplex™ NG & DR Assay* is a multiplex real-time PCR assay that can accurately detect NG and drug resistance (Azithromycin, Ciprofloxacin) with a single test. It can also be used with Seegene’s other STI assays to provide informative STI testing results.
Mycoplasma genitalium (MG) has been recognized for potentially serious health complications including urethritis in men, and cervicitis, PID, preterm birth, and spontaneous abortion in women. Primary treatment of MG is the macrolide antibiotic azithromycin. For azithromycin-resistant MG, the fluoroquinolone antibiotic moxifloxacin can be used as the second-line treatment. For M. genitalium, drug resistance is an emerging problem similar to other STIs. Some strains exhibit resistance to both lines of treatment with resistance easily acquired through single point mutation in the DNA.
Seegene offers two drug-resistance detection assays for M. genitalium. Both assays detect single DNA mutations that cause resistance as well as MG infection itself. Both assays use exogenous IC for urine and endogenous IC for others. The Novaplex™ MG & AziR Assay* and the Novaplex™ MG & MoxiR Assay* are designed to be run together with the Novaplex™ CT/NG/MG/TV Assay*.
*Novaplex™ Assays are For Research Use Only. Not for Use in Diagnostic Procedures.
1 Dalhoff A. (2012). Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdisciplinary perspectives on infectious diseases, 2012, 976273. https://doi.org/10.1155/2012/976273 2 (image) Gonococcal Isolate Surveillance Project (GISP) - Gonorrhea - STD information. Centers for Disease Control and Prevention. (2022). Retrieved from https://www.cdc.gov/std/gisp/default.htm


